Natalie Chrystal and Rudolf van der Veen
Introduction
Moulds are found through the world with Diekman and Long (1988) reporting that 200 000 species of moulds have been identified. Fortunately, although all moulds produce primary metabolites in order to synthesise biomass and to generate energy for primary metabolism, only a few species (and even strains) of mould produce secondary metabolites, mainly after a phase of balanced growth and usually in association with a morphogenetic change such as sporulation (Leeson and Summers, 2001). Mycotoxins, harmful to animals and humans, fall into this group of secondary metabolites, which also includes pigments and compounds active against micro-organisms (antibiotics) or plants (phytotoxins) (Lesson and Summers, 2001).
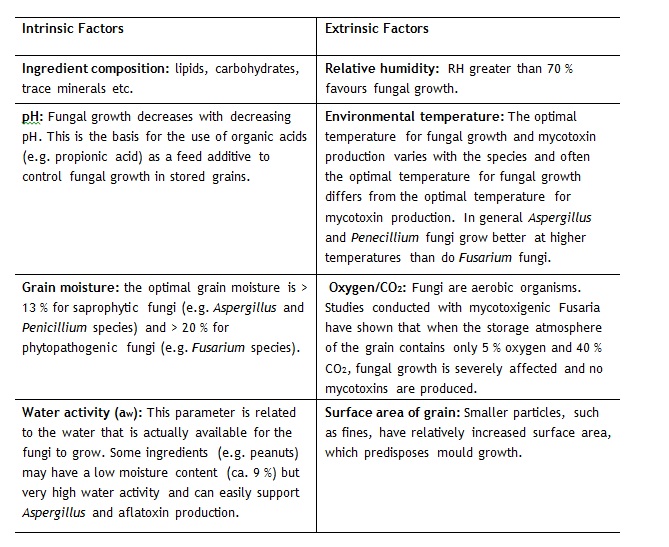
Moulds reproduce by means of asexual reproduction and the subsequent formation of spores which spores are spread through the air or by means of insect vectors. Under favourable environmental conditions mould spores will germinate, grow and reproduce as they utilise nutrients present in the grain and mycotoxin production may then occur. As a consequence of the relative ease with which mould spores can be spread, mould spores, mould itself and mycotoxins are a common contaminant of those raw materials frequently used in feed manufacture (Hundley 2001). However, both Pittet (2001) and Leeson and Summers (2001) reported that mould growth and subsequent mycotoxin production is highly dependant on environmental or extrinsic factors with Lesson and Summers (2001) further reporting that intrinsic factors relating to the substrate itself also play an important role in the potential for mould growth and mycotoxin production. The factors considered important by Leeson and Summers (2001) are given in table 1.
Mould and Mycotoxin classification
Mycotoxigenic moulds found to contaminate grain have traditionally been classed as either phytopathogenic moulds, which attack the plant in the field or saprophytic moulds, which preferentially infect stored grain (Placinta et al., 1999; Leeson and Summers, 2001), although certain mould species may fall into both categories. Moulds belonging to the species Aspergillus and Penicillium are common examples of saprophytic fungi whilst Fusarium species are an example of mycotoxigenic phytopathogens (Leeson and Summers, 2001). A. flavus however may behave as both a saprophytic and phytopathogenic mould, infecting both stored grains and crops in the field (Placinta et al., 1999). These three species of fungi are the main mycotoxin producing mould species.
A wide range of mycotoxins, with a diversity of chemical structures and biological activities, have been identified with Buckle and Scudamore (1990) reporting that several hundred mycotoxins have been described. However, the majority of these have only been reported under laboratory conditions and only about 30 different mycotoxins have been reported to occur naturally, in a wide range of feedstuffs, which have undergone fungal spoilage (Buckle and Scudamore, 1990). Of the known mycotoxin groups, aflatoxins, ochratoxins, trichothecenes, zearalenone and fumonisins are generally considered to be the most important, in animal production (Henman, 2003).
Table 1: Factors affecting mould growth and mycotoxin production in cereal grains (Leeson and Summers, 2001)
Control and prevention of mould growth and mycotoxin production
In his review Pittet (2001), noted that commodities can be come contaminated with mycotoxins at any time in the production cycle, i.e. at any stage from growth in the field through harvesting, processing, storage and shipment. He further noted that as the fungal flora is likely to differ significantly at each stage there is the potential for a number of different mycotoxins to develop through the life of an agricultural commodity if proper care is not taken in the production, handling and storage of the crop. This view would agree with that of Hundley (2001) and AFMA (2003) who noted that there are three to five (depending on possibly grouping of phases) distinct phases that make up the production cycle and during which fungal colonisation can possibly take place. They are:
- Pre-harvest
- Harvesting
- Storage
- Feed Manufacture
- Livestock Production
Clearly not all of these production phases are under the control of the feed manufacturer but treatment and processing of grain prior to acquisition by the feed manufacturer, should be important criteria in the purchasing of raw materials. A brief review of recent developments in this area will be discussed in this paper. However, both the reports of Hundley (2001) and AFMA (2003) further describe those measures which can and should be taken at each stage in the production cycle in order to minimise the risk of mould growth and mycotoxin production and these reports should be referred to for further detail.
Control measures for raw materials during growth and harvest
The feed manufacturer has little control over the agronomic practices in place on those farms from which crops are purchased. However, with the advent of traceability and other quality assurance procedures such as GMP it is not unrealistic for feed manufacturers to request that some background into the practices associated with crop production are provided. As AFMA (2003) noted, it is imperative that all involved in the production cycle are involved in the control of mycotoxin production and accept responsibility for their part in the cycle.
Recent work carried out in Europe (Oldenburg and Brunotte, 2002), along with some circumstantial evidence (Markert, 2003, pers. comm.) suggests that practices such as zero and minimum tillage have resulted in an increase in the DON content of the wheat crop. So, whilst these agronomic practices are recognised to have benefits for soil conservation feed millers should at least be aware of the potential increase in mycotoxin levels as a result.
In addition, whilst there is considerable evidence that the use of fungicides in field, help to prevent and reduce mould growth, there is new evidence showing that the use of strobilurin fungicides containing kresoxym-methyl or azoxystrobin has resulted in an increase in the DON content of crops (Obst and Gammel, 2000; Oldenburg and Brunotte, 2002). It is thought that although strobilurins, a group of chemical fungicides commonly used in Europe to control head blight in wheat, have been shown to be relatively effective against Fusarium ear blight, it appears that certain classes of strobilurins may be more selective than others and whilst preventing that growth of certain Fusarium strains, they do not inhibit the growth of others, with the subsequent result that mycotoxins such as DON produced by these moulds
Whilst it is commonly recognised and accepted that grains, which appear to be mould free, may not be free of mycotoxins, it is also important to note that not all strains of a particular species of fungus are mycotoxigenic (Leeson and Summers, 2001). These authors noted that in the case of A. flavus, for example, only about 10 % of the strains are capable of producing aflatoxin and therefore contamination of a given substrate with a fungus does not necessarily imply mycotoxin contamination.
Whilst certain control measures can be implemented by producers, it is worth noting that Park and Liang (1993; cited by Pittet, 2001) observed that although good crop management techniques go some way to reducing the problem of mycotoxins, they never provide a complete solution.
Control measures for raw materials during storage
The prevention of mould growth and mycotoxin production during storage requires a multifactorial approach and a number of control measures related to both the crop itself and to the storage facilities can be implemented in order to reduce the potential for colonisation. The same principals applied during storage should also apply to the transport of the grains. Cleaning of the vehicles and associated machinery is particularly important to prevent cross contamination of grains, whilst the treatment of grains with a mould inhibitor prior to transport is particularly important where grains are to be transported long distances (Leeson, 1997).
Control measures related to the facilities themselves include regular cleaning and treatment with mould inhibitors, especially in areas referred to commonly as “dead spots” (AFMA, 2003).
Where raw materials are concerned, acceptation of these should be on the basis that certain control criteria are met. Where the set criteria are not met appropriate action should be taken.
Moisture content is a common parameter that is measured by feed millers prior to a crop being accepted for storage, although a more accurate measure of the potential for mould growth may be water activity (aw), which refers to the unbound water found in food (or feed) and which may support the growth of bacteria, yeasts and moulds (Food Science Australia, 2000). Water activity, is measured on a scale of 0 to 1, with 0 being very dry and 1 being the aw of pure water (Food Science Australia, 2000). Water activity is not the same as moisture content of feedstuffs and whilst moist foods (or feeds) tend to have a higher aw than dry feeds this is not always the case (Food Science Australia, 2000).
Below an aw value of 0.6 there is no risk of microbial or fungal deterioration of the feed (unless insects are present) as the water present in the feed is bound to the starch and protein molecules of the raw material itself. However, where insects are present they may digest the feed resulting in the formation of CO2 and free water leading to an increased aw value and the possibility of microbial or fungal spoilage (van Wesel, 2002).
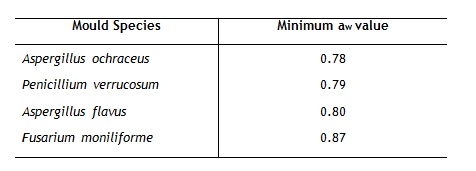
Between aw values of 0.6 and 0.9 mould growth may occur whilst above an aw value of 0.9 (visible free water is present) bacterial growth may occur. Above an aw level of 0.6, a mould inhibitor should be used. Some minimum aw values required growth of toxigenic mould species are given in table 2 (WHO, 2000).
Table 2: aw value for growth of toxigenic mould species
Where free water is present condensation of this free water may occur during storage. In cooler spots the aw value may thus rise above 0.6 causing localised mould or bacterial growth. An average aw value of 0.6 therefore should be treated with caution (van Wesel, 2002).
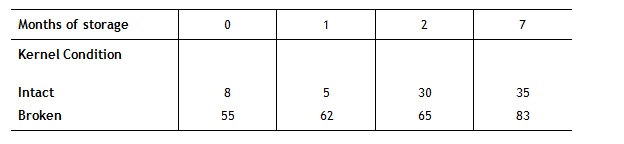
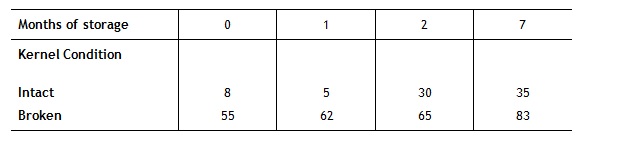
Turnaround time of stored grains is another area, which could possibly receive more attention. Whilst the best policy for use of raw materials in the feed industry may appear to be a first in, first out policy, there will always be some variability in the raw materials which are received by a feed mill and where possible some allowance should be made for this. For example, Cook (1991) showed that mould growth following storage is considerably higher in grains, which are cracked or broken than in those, which are intact. In such an instance using cracked grains prior to whole grains is likely to go some way towards preventing the build of moulds in storage bins and potential mycotoxin production.
Table 3: Mould growth (% mould) on intact and broken kernels after storage (Cook, 1991)
Control measures during feed manufacturing
As with feed storage facilities regular cleaning to prevent the build up of organic material plays an important role in the production of “clean” feed. Furthermore, regular control of manufacturing procedures and the implementation and close following of programmes such as GMP and HACCP will help to reduce the risk of mycotoxin contamination of feed leaving the mill. The review of Hundley (2001) covers this topic comprehensively.
Control measures during livestock production
Although feed manufactures can take a number of measures to ensure that feed relatively free of mycotoxins leaves the mill, this feed is likely to spend a fair amount of time in storage on the farm prior to consumption by the animals. There is little the feed miller can do to prevent this aside from supplying sound information related to the storage of feed and including a mould inhibitor where required.
Mycotoxin sampling, detection and testing
Regular sampling of “suspicious” or high-risk raw materials is extremely important and complete feeds should routinely be tested for the presence of mycotoxins. There are a number of methods, which can be used to detect the presence of mycotoxins and additional methods allowing for the quantification of mycotoxin levels. However, discussion of this topic is beyond the scope of this review. Suffice to say that particular attention should be given to the drawing of samples for mycotoxin analysis, as a large proportion of the variation in analytical results where mycotoxins are concerned can be attributed to sampling methods.
Four main methods for detecting either the fungi that produce mycotoxins or mycotoxins themselves have been identified and reported by Diekman et al. (1997) and these are visual inspection, the use of black light, immunoassays and chromatography. However, none of these methods are entirely fool proof and results obtained should be interpreted with the necessary mix of caution and practical knowledge.
Reducing the effects of mycotoxins in the feed on animal performance
Although a number of methods can be used to prevent mould growth and mycotoxin production, it is almost impossible for the feed miller to prevent all mould contamination and subsequent mycotoxin contamination of feed. Consequently most feed ingredients derived from plant sources are likely to be contaminated with mould spores, mould or mycotoxins to some degree or another. Good manufacturing practices therefore aim to ensure that feeds contain the lowest possible amounts of these contaminants. Furthermore, the variability associated with current methods of mycotoxin detection, interaction between mycotoxins and the virtual absence of legislated maximum limits for mycotoxins means that mycotoxicosis is still a common problem in animal production. Of perhaps more concern, are recent research results published by Oswald et al. (2003) who suggested that all levels of the immune system are affected by mycotoxins at inclusions much lower than those that result in physical signs of mycotoxicosis. The consequences of the broad immuno-suppressive effect of mycotoxins include increased susceptibility to infection, increased severity of infection and increased risk of vaccination failure and these will have a significant impact on the ability of the animal to achieve its genetic potential.
Numerous authors have reported that the feeding of grains naturally contaminated with mycotoxins appears to result in a greater toxicity than the feeding of diets containing an equivalent amount of purified mycotoxin. This observation is likely to be a result of the fact that naturally contaminated grains contains more than one mycotoxin and the interaction between these results in greater toxicity than would either mycotoxin on its own. Thus as Smith et al. (2001) noted research which takes account of such interactions (mainly by the use of naturally contaminated grains rather than purified mycotoxins) will go a long way in validating the manufactures claims of in vitro and in vivo binding capacities of a toxin binder as well as other solutions to the problems of mycotoxins.
As a result of the complexity of the problem posed by mycotoxin contamination of animal feeds, as well as the availability of more research data related to the effects and interactions between mycotoxins themselves, the animal and nutrients contained in the feed, more recent means for counteraction have moved beyond simply preventing fungal growth in field and stored grains. Current emphasis is thus on reducing the deleterious effects of the mycotoxins in feeds while enhancing production and improving immune response and there are a number of possibly ways in which this can be achieved.
The following paragraphs will provide a brief overview of the different methods that are currently in use and which may become more widely used in the future as a means of controlling the threat posed to animal production by mycotoxins.
Decontamination
Research into various means of removal of mycotoxins from contaminated grain has been conducted and a number of possible processes have been investigated. Ellis et al. (1991; cited by Leeson et al., 1995) reviewed possible methods for the decontamination of aflatoxin-contaminated feed and these included organic solvent extraction, physical separation, heat treatment, irradiation and ammoniation. It has been suggested that this last process is the most reliable on farm method for the detoxification of grains contaminated with aflatoxin (Leeson et al., 1995) with Park (1993; cited by Leeson et al., 1995) reporting a 99% reduction in aflatoxin levels as a result of ammoniation of aflatoxin contaminated corn, cottonseed, cotton-seed meal and peanut meal. However, these authors also noted that this method is hazardous, toxic and very corrosive. In addition, although the technology exists, there is as yet, no practical method to economically decontaminate large volumes of mycotoxin-contaminated grains (Diekman et al., 1997; Leeson, 1995; Leeson et al., 1995). The use of hydrogen peroxide as an oxidizing agent has also been reported to result in a 97% reduction of aflatoxin levels in contaminated grain and peanut meal (Ellis et al., 1991; cited by Leeson et al., 1995). Other methods of decontamination for aflatoxin-contaminated grains include the use of organic acids such as isobutyric and propionic-acetic acid and the use of surfactants (Leeson et al., 1995).
Dilution
Dilution of mycotoxin-contaminated grain, containing levels of mycotoxin above those recommended as safe, with grain free of mycotoxins, is a common solution to the problem of mycotoxins. However, there are a number of problems associated with this method of control in that “safe levels” for mycotoxins in feed are still extremely uncertain and as mentioned earlier it is possible that mycotoxins exhibit effects on the immune system at levels far lower than those which result in clinical symptoms of disease. Furthermore, as from the 1st of August 2003, this practice is no longer allowed in the EU where grain is contaminated with aflatoxin B1 (EU Directive 2003/32/EU). As yet no other mycotoxins are included on the EU list of undesirable substances, however there is little doubt that overtime more and more mycotoxins will be added to the undesirable substances list and the same legislation will then apply.
Use of Mould inhibitors
To maintain and ensure a good feed quality many feed manufacturers include a mould inhibitor in the feed. However, if applied at a low level they tend to loose their effectiveness after three to four days. In addition, these inhibitors may not be able to prevent or reduce growth in areas where the moisture level in the bin has increased through water migration (CSIR Food and Technology, 1997).
The use of mould inhibitors as a means of curbing fungal growth has its origins many decades ago with the use of copper sulphate and gentian violet as inhibitors. However, the technology associated with this practice has evolved considerably and there are now a number of potent anti-fungals available for commercial use. The main types of mould inhibitors currently available include combinations of or individual organic acids (such as propionic, sorbic, benzoic and acetic acids), salts of organic acids (such as calcium propionate and potassium sorbate) and copper sulphate, with the two former types being the most readily used in the feed industry (Jones et al., 1994; CSIR Food and Technology, 1997).
Organic acids, with a low molecular weight, are an important class of fungistats, which inhibit fungal growth (Jones et al., 1994), although they cannot inactivate any of the toxins already present in the grain. Salts of organic acids tend to be longer lasting and less corrosive than the more volatile free acids, although the free acids have a greater ability to penetrate into the stored grain and thus better anti fungal activity.
When using mould inhibitors the following points should be considered.
- Dispersion: A mould inhibitor can only be effective if it is completely and thoroughly distributed throughout the feed. Ideally this means that the entire surface of each feed and or grain particle should come into contact with the inhibitor and that the inhibitor should also penetrate feed particles so that the moulds colonising the interior of the grain will be inhibited (Jones et al., 1994). Thus, the surface area of powdered mould inhibitors should be as large as possible (CSIR Food Science and Technology, 1997). Liquid mould inhibitors are commonly used in silo’s where they can be applied using a spray. They may also be applied post pelleting.
- Effect of feed ingredients: Certain feed ingredients can affect the performance of mould inhibitors. Protein or mineral supplements such as soyabean meal, fishmeal, poultry by product, and limestone tend to reduce the effectiveness of proprionic acid. These materials can neutralise free acids and convert them to their corresponding salts, which are less active as inhibitors (Jones et al., 1994; CSIR Food Science and Technology, 1997).
- Time dependence: Mould inhibitors used at manufacturers recommendations will produce a period of freedom from mould activity. If a longer mould free period is required a higher concentration of inhibitor should be used. The concentration of the inhibitor begins to decrease almost immediately after it is applied as a result of chemical binding, mould activity or both. Furthermore feeds that are heavily contaminated with moulds require extra amounts of inhibitor to achieve the desired level of protection (Jones et al., 1994; CSIR Food Science and Technology, 1997).
Mycotoxin binders
Possibly the most common range of products currently available on the market for the control of mycotoxins in feed are those products falling under the broad category of mycotoxin binders. These products can be largely subdivided into three separate groups namely, clays and zeolites, activated charcoals and yeast cell walls.
Taylor (1999) reported that a large amount of work has been carried out on the binding efficacy of both inert materials and inorganic mycotoxin binders, although most of work has focused on the ability of the binders to bind aflatoxins, particularly aflatoxin B1 as this is the mycotoxin for which in vivo binding efficacy has firmly been established.
O’Sullivan (2003; cited by Mellor, 2003) suggested that a good mycotoxin binder should
- absorb a range of mycotoxins
- have a low effective inclusion in the feed
- show rapid and even dispersion in the feed during mixing
- be heat stable during pelleting and processing
- not bind other in-feed nutrients such as vitamins and minerals
- have a high stability at a wide range of pH
- be biodegradable following excretion
In addition the binding properties observed in vitro, i.e. total and stable binding as well as in vivo efficacy are extremely important properties of any binder. Smith et al. (2001) observed that stable binding is a more important measure of efficacy than total binding as variation in gut pH, gut contents and the effect of enzymes amongst other factors may influence the stability of the bound complex. If the binder-mycotoxin complex is not stable the binder may release free toxins in lower parts of the GIT and these will become available to the animal for absorption (Smith et al., 2001).
When comparing the efficacy of binders it is important to have a complete picture of the conditions under which the binder was evaluated, as these will all have a considerable impact on the binding capacity of any given binder. Experimental factors, which should be considered, include binding conditions such as time, temperature, solvent and binder particle size, concentration of the binder and that of the toxin and the detection methods used in analysis (Taylor, 1999).
Clays and zeolites: Clay and zeolitic minerals are a diverse and complex range of aluminosilicates with a wide variety of functional properties (Phillips et al., 1988). In general however, clays possess a distinctive layered structure whilst zeolites posses a highly porous three-dimensional network of interconnecting pores (Taylor, 1999). Both these mineral classes are made up of at least 30 different naturally occurring variants each possessing specific and characteristics differences in their chemical and structural make up (Taylor, 1999).
Hydrated Sodium Calcium Alumino Silicates: Hydrated sodium calcium alumino silicates (HSCAS) is a broad grouping of clays and zeolites (both of which can be classed as aluminosilicates) which contain some internal water of crystallisation and lesser amounts of sodium and calcium in the form of exchangeable cations or their soluble salts (Taylor, 1999). However as a result of the broad nature of this classification, there is considerable variation in the structure and chemical composition of this class of compounds. Consequently not all HSCAS are the same with respect to their ability to bind mycotoxins and the subsequent stability of this bound complex. Binding ability and stability of any HCSAS will be dependant on surface pH, pore size and availability of exchangeable cations, amongst others (Taylor, 1999). It is therefore of utmost importance to establish the binding ability and stability of HSCAS before it as used as a toxin binder.
Phillips et al. (1988) showed HSCAS to have a high affinity for aflatoxin B1 in vitro following the screening of 38 different adsorbents representative of this class of minerals. These authors further observed that the inclusion of an HSCAS at 0.5 % in a diet containing 7.5 mg kg-1 of aflatoxin B1 resulted in an improvement in growth performance of broiler chicks. However, the European Mycotoxin Network (2003) reported that the efficacy of HSCAS against zearalenone, and ochratoxin A is limited and that it is virtually ineffective against tricothecenes such as DON and T-2 toxin.
Bentonites: Sodium Bentonite has been used as a binder and lubricant in pelleted feed for some time (EMAN, 2003). Claims relating to the ability of bentonite to bind aflatoxin have been made, although several in vivo experiments reviewed by Ramos et al. (1996; cited by EMAN, 2003) gave contrasting results and proved inconclusive. In their review these authors also reported that although effective against T-2 toxin in rats, bentonite was ineffective against zearalenone and nivalenol in pigs.
Activated Charcoal: Activated charcoal is the residue derived from the destructive distillation of organic matter of vegetable origin (Leeson et al., 1995) and shows different adsorbing properties depending on its origin (EMAN, 2003). It has a high binding capacity and the efficiency of adsorption is unaffected by the pH of the toxin, with the adsorbed material usually retained through the entire GIT (Osweiller et al., 1985; cited by Leeson et al., 1995).
Although it is generally accepted that activated charcoal will bind both aflatoxin and ochratoxin A successfully, reports relating to the efficacy of activated charcoal as an aflatoxin binder are varied and contradictory (Leeson et al., 1995; EMAN, 2003) while Rotter et al. (1989; cited by Marquardt and Frohlich, 1992) reported that activated charcoal was an impractical method of reducing ochratoxin A toxicity in chicks continuously receiving ochratoxin A. Edrington et al. (1997) investigated the efficacy of super activated charcoal as a binder for T-2 toxin and these authors concluded that although performance results were largely variable over trial, the efficacy of super activated charcoal in reducing some of the toxic signs of chronic aflatoxicosis was marginal in growing broilers but was of little benefit where T-2 toxins were fed.
Thus, as Leeson et al. (1995) although adsorption therapy (i.e. the adsorbtion of ingested toxins) has become one of the most important methods of alleviating mycotoxicosis these absorbents also have the capacity to bind in feed medications and other nutrients such as vitamins and minerals. As a consequence the inclusion of such binders in feeds needs to be carefully evaluated and the potential loss of beneficial nutrients weighed up against the benefit of the mycotoxin removal.
Yeast Cell walls: Stanley et al. (1993; cited by Aravind et al., 2003) reported that Saccaharomyces cerevisiae was found to alleviate the adverse effects of mycotoxins in poultry and these beneficial effects were later attributed to the presence of esterified glucomannans derived from the cell wall of Saccaharomyces cerevisiae1026 (Aravindet al., 2003). Subsequently esterified glucomannans were shown to be effective in binding several commonly occurring mycotoxins such as aflatoxin, ochratoxin A, zearalenone and T-2 toxin (Devegowda et al., 1998). Recent research by Aravind et al. (2003) lead these authors to conclude that the addition of esterified glucomannan at 0.05 % may be sufficient to counteract the adverse effects of mycotoxins in a poultry fed a diet naturally contaminated with 168 ppb of aflatoxin, 8.4 ppb of ochratoxin A, 54 ppb of zearalenone and 32 ppb of T-2 toxin. However, these authors also noted that the precise mode of action of esterified glucomannans is not known although irreversible entrapment of mycotoxins (Devegowda et al., 1996; cited by Raju and Devegowda, 2000), stimulation of the immune system (Savage et al., 1996; cited by Raju and Devegowda, 2000) alteration of intestinal microbial environment (Newman, 1994; cited by Raju and Devegowda, 2000) and provision of nutrients beneficial to gut flora (Raju and Devegowda, 2000) have all been proposed.
Microbial and enzymatic detoxification methods
From the data presented above it can be seen that a large amount of the data currently available on mycotoxin binders suggests that these products may provide a solution where contamination with aflatoxin and in some cases ochratoxin A has occurred. However the efficacy of these products in binding the other common mycotoxins, such as zearalenone and DON, is limited. Consequently alternative strategies for coping with less adsorbable mycotoxins are required.
Van der Eijk (2003; cited by Mellor, 2003) reported that degradation or transformation of mycotoxins by means of enzymes has been shown to be a successful means of detoxifying the mycotoxins such as T-2 and zearalenone respectively. Mellor (2003) further noted that removal of the 12,13 epoxide ring of tricothecenes using de-epoxidse enzymes results in a significant reduction in toxicity. It well known that of all commonly farmed livestock species, ruminant animals are frequently less susceptible to the threat of mycotoxins than the monogastric animals and one of the first microbial organisms developed as a feed additive used to degrade mycotoxins was identified in bovine rumen contents (Binder et al., 2001; cited by Mellor, 2003). Fuchs et al. (2003) reported that this bacterial strain (BBSH 797) has the ability to transform DON into a non-toxic de‑epoxide DOM-1. In addition these authors reported that six additional type A tricothecenes were seen to be transformed. An Austrian based company BiominÒ has patented BBSH 797 as a tool for the prevention of mycotoxicosis in animal production.
Nutritional and management strategies
Two possible strategies to control the effects of mycotoxins, which should not be overlooked, are those of dietary manipulation and on-farm management.
Interactions between a number of different nutrients and the effects of mycotoxins have been reported. The manipulation therefore of nutrient levels in diets may help the animal to overcome mycotoxicosis. Such strategies will vary depending on the mycotoxin and its effect on the animal, but may include strategies such as increasing antioxidant (BHT) levels in the diet (Ehrich et al., 1986; cited by Leeson et al., 1995) or increasing the level of dietary selenium (Leeson et al., 1995) where aflatoxicosis is a problem or increasing dietary protein levels and supplementing with vitamin C (Leeson et al., 1995) where ochratoxicosis is likely to be a problem. However, strategies for combating the ill effects of one mycotoxin will not necessarily be effective against others, as Hoehler and Marqhardt (1996; cited by D’Mello et al., 1999) reported that supplementation with vitamin C was ineffective against the effects of T-2 toxin. Supplementation with vitamin E did however go some way towards reducing in vivo lipid peroxidation in chickens suffering from T-2 toxicosis (Hoehler and Marqhardt, 1996; cited by D’Mello et al., 1999).
The effects of mycotoxins vary considerably between animal species and different age groups of animals. In addition the effects are also dose and duration of exposure dependant and are usually more severe in very young animals or animals exposed to a disease challenge. Consequently, as a last resort, reducing the period of time over which animals are exposed to mycotoxins in the diet or feeding grains contaminated at low levels to less susceptible animals may help to reduce the “overall” effects of mycotoxins.
As described above a large number of products, aimed at reducing the effects of mycotoxins in animal feeds, are currently available on the market. However, as with any feed additive, the claimed efficacy of these products and the mode of action should always be considered carefully prior to their inclusion in feed. Although there does not appear as yet to be one single solution to the problem of mycotoxin contamination of feed and subsequent mycotoxicosis, more and more information about mycotoxins and their effects in animals is constantly becoming available along with improved methods of analysis, thereby allowing the development of new products and strategies which can be used to effectively reduce (or prevent) the harmful effects of mycotoxins in animal feeds (Gerber, 2003).
Conclusion
In order to effectively manage the threat of mycotoxins, the entire supply chain for animal feed must be involved. Effective quality control methods such as HACCP and GMP along the length of the chain should be implemented to reduce the potential for mould growth and subsequent mycotoxin production. However, as a result of variability associated with the production and structural and physical properties of mycotoxins it is unlikely that a single solution for all mycotoxins will apply and some flexibility will be required in the approach adopted to deal effectively with the problem of mycotoxins.
- References available on request
- This paper was first published in the Proceedings of the AFMA Myctoxin Workshop, 2003.